Abstract
Background: HLA-B leader encodes methionine (M) or threonine (T) at position 2 and gives rise to TT, MT, or MM genotype. HLA-B M leaders promote higher HLA-E expression than T leaders, enhancing T cell and natural killer (NK) cell recognition on HLA-E via NKG2A and NKG2C related to cytomegalovirus (CMV) recognition. The dimorphic HLA-B leader informs acute graft-versus-host disease (GVHD) risk in HLA 1 allele mismatched unrelated hematopoietic stem cell transplantation (HCT) (Petersdorf EW et al. Lancet Haematol. 2020 and Blood. 2020) and haploidentical HCT (Fuchs EJ et al. 62nd ASH Annual Meeting 2020). However, the impact of the HLA-B leader genotype in HLA-matched related/unrelated donor HCT from the viewpoints of CMV reactivation has not been elucidated fully yet. We performed this retrospective study to explore the significance of HLA-B leaders in HLA-matched related/unrelated HCT in the Japanese population.
Methods: All clinical data of 10,110 patients who underwent 8/8 HLA matched related/unrelated donor bone marrow/peripheral blood HCT for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndrome (MDS) between 1996 - 2019 were provided by the Japanese Data Center for Hematopoietic Cell Transplantation. All 8 alleles at HLA-A, -B, -C, and DRB1 were matched by genotyping, and sibling pairs whose genotype were unknown but matched 8/8 for HLA antigen at a serologic level were included. Study outcomes were overall survival (OS), relapse, non-relapse mortality (NRM), grade II-IV acute GVHD, grade III-IV acute GVHD, and chronic GVHD. Multivariable models using Cox regression analysis assessed transplant outcomes associated patient age, patient sex, patient performance status, donor sex, donor age, diagnosis, disease risk index (DRI), donor source (bone marrow or peripheral blood), related/unrelated donor, myeloablative (MAC)/reduced-intensity conditioning, patient CMV serostatus and patient/donor HLA B-leader (TT, MT, MM). In subgroup analysis, we adopted results of CMV antigenemia instead of CMV serostatus to evaluate the impact of CMV reactivation for HCT outcomes. All statistical analyses were performed with EZR.
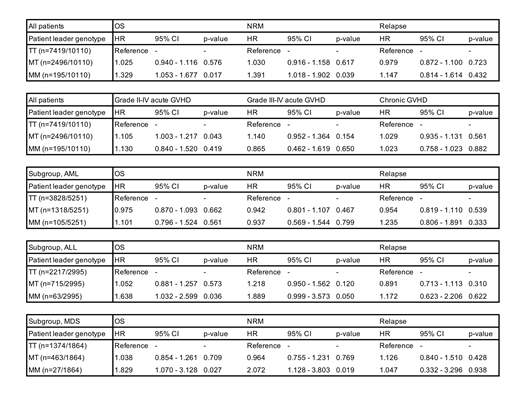
Results: This study included 5,212 AML patients (51.9%), 2,995 ALL patients (29.6%) and 1,864 MDS patients (18.4%). In DRI, low risk was 501 (5.4%), intermediate risk was 5,750 (61.6%), high risk was 2,711 (29.0%) and very high risk was 378 (4.0%). Median patients age was 44 (range 0-77) years. Bone marrow was the graft source in 7,183 recipients (71.0%). Related donors were 5,378 (53.2%). MAC was used in 5,891 (70.3%) patients. The number of TT patients/donor was 7,419 (73.4%), MT patients was 2,496 (24.7%) and MM patients was 195 (1.9%). MM patients was associated with significant lower OS (hazard ratio [HR] 1.329 [95% CI, 1.053 - 1.677]); p = 0.017 and higher NRM (HR 1.391, [95% CI, 1.018 - 1.902]); p = 0.039) compared to TT patients (Table). There was no significant correlation between MM patients and grade II-IV/III-IV acute GVHD. In subset analysis for each diagnosis, MM genotype didn't affect outcomes in AML patients, whereas MDS and ALL patients with MM genotype showed lower OS (MDS: HR 1.829, [95% CI, 1.070 - 3.128]; p = 0.023), (ALL: HR 1.638, [95% CI, 1.032 - 2.599]; p = 0.037) compared to TT genotype (Table). In subgroup analysis for HLA-B leader genotype, CMV reactivated patients were significant better for OS (HR 0.467, [95% CI, 0.266 - 0.819]; p < 0.001) and lower NRM (HR 0.342, [95% CI, 0.153 - 0.763], p = 0.009) only in MM patients.
Conclusions: MM HLA-B leader genotype is a risk factor for worse OS and higher NRM compared to TT genotype in HLA matched related and unrelated HCT, particularly MDS and ALL patients in the study. On the other hand, CMV reactivation could be favorable for OS and NRM in MM leader patients suggesting that promoting NK cell reconstitution and education due to CMV reactivation might benefit MM leader patients.
Kanda: CHUGAI PHARMACEUTICAL Co., Ltd.: Honoraria; DAIICHI SANKYO Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Eisai: Research Funding; Janssen Pharmaceutical K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin Co., Ltd.: Honoraria; Megakaryon Co: Honoraria, Membership on an entity's Board of Directors or advisory committees; NextGeM Inc: Patents & Royalties; Novartis Pharma K.K.: Honoraria; Ono Pharma Inc.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Sanofi K.K.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; SymBio Pharmaceuticals, Ltd.: Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceutical Company Limited: Honoraria, Membership on an entity's Board of Directors or advisory committees; TEIJIN PHARMA LIMITED.: Honoraria; Bristol-Myers Squibb Co: Honoraria; Astellas Pharma Inc.: Consultancy, Honoraria; Amgen Astellas BioPharma: Honoraria. Ichinohe: Takeda Pharmaceutical Co.: Honoraria; Kyowa Kirin Co.: Honoraria, Research Funding; FUJIFILM Wako Chemicals.: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; CSL Behring: Honoraria, Research Funding; Taiho Pharmaceutical Co.: Research Funding; Sumitomo Dainippon Pharma Co.: Honoraria, Research Funding; Ono Pharmaceutical Co.: Honoraria, Research Funding; Nippon Shinyaku Co: Research Funding; Takara Bio Inc.: Research Funding; Zenyaku Kogyo Co.: Research Funding; Repertoire Genesis Inc.: Honoraria, Research Funding; Novartis Pharma K.K.: Honoraria; Celgene: Honoraria; Otsuka Pharmaceutical Co.: Research Funding; Eisai Co.: Honoraria, Research Funding; Chugai Pharmaceutical: Research Funding; Bristol-Myers Squibb: Honoraria; AbbVie Pharma: Research Funding; Astellas Pharma: Honoraria, Research Funding. Atsuta: Mochida Pharmaceutical Co., Ltd.: Speakers Bureau; Meiji Seika Pharma Co, Ltd.: Honoraria; Astellas Pharma Inc.: Speakers Bureau; AbbVie GK: Speakers Bureau; Kyowa Kirin Co., Ltd: Honoraria.